Who regulates Neuroscience Products at the Food & Drug Administration (FDA)?

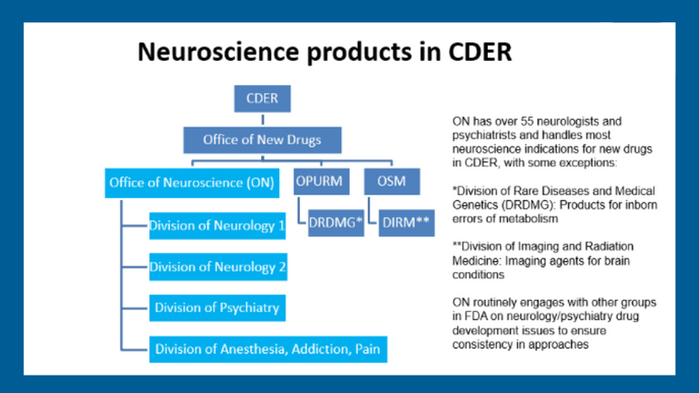
Neuroscience products in CDER
Neuroscience products at CBER
Neuroscience products at CDRH
Recent FDA Guidelines
Early Alzheimer's Disease: Developing Drugs for Treatment (2024)
Migraine: Developing Drugs for Preventive Treatment (2024)
Psychedelic Drugs: Considerations for Clinical Investigations (2023)
Clinical Considerations for Studies of Devices Intended to Treat Opioid Use Disorder (2024)
Human Gene Therapy for Rare Diseases (2020)
Human Gene Therapy for Neurodegenerative Diseases (2022)
FDA Regulatory Science Initatives
Complex Innovative Trial Design Meeting Program
Critical Path INnovation Meetings (CPIM)
Fit-for-Purpose Initiative
Model-Informed Drug Development Program
Real World Evidence Program
Digital Health Center of Excellence
Quantitative Medicine Center of Excellence
CDER Center for Clinical Trial Innovation (C3TI)
Cross-Center Collaboration
-
Neurology across FDA meetings
-
Quarterly meetings of review office leadership from CBER, CDER, and CDRH
-
Share knowledge and information among FDA Centers regarding scientific, regulatory, and other issues related to neurology
-
Enhance collaboration and cooperation across all three medical product centers
-
Provide opportunities for reviewer training (e.g., Intracranial delivery of novel gene and cell-based therapies)
-
Accelerating Access to Critical Therapies for ALS Act (ACT for ALS)
-
FDA Rare Neurodegenerative Diseases Task Force
-
Intercenter collaboration to address common scientific, clinical, and policy issues related to rare disease product development
Neurology Drug Program
ABC's Role
The American Brain Coalition (ABC) plays a leading role in advocating for the importance of FDA neuroscience including the Neurology Drug Program, demonstrating its commitment to advancing neurological and psychiatric research, treatment, and care within the United States. As a leading coalition of organizations dedicated to improving brain health, ABC leverages its collective voice and expertise to champion this initiative to support individuals living with brain diseases and conditions.
Through strategic collaborations with policymakers, healthcare professionals, researchers, and patient advocacy groups, ABC effectively advocates for increased funding for the Neurology Drug Program to accelerate the development and approval of new therapeutics for individuals with brain diseases and disorders.
How to Participate
American Brain Coalition convenes a working group of organizations committed to advancing the Neurology Drug Program. We also encourage you to join and share the American Brain Coalition's Neurology Drug Program Fiscal Year 2025 sign-on letter requesting funding for the FDA to advance the Neurology Drug Program and help speed the approval of safe and effective treatments for individuals who need them.
Your support is crucial in advocating for resources that will enable the development of innovative treatments and therapies for individuals living with brain diseases and conditions. By engaging in this advocacy effort, we can work towards improving the quality of life for countless individuals and families affected by these conditions. Thank you for your commitment to this important cause.
Please reach out to Katie Sale if your organization would like to join this effort.
ABC Activity
.png)